
INSTI HIV-1/HIV-2 Anitbody Test
The INSTI HIV-1/HIV-2 Antibody Test is a one-time use, rapid, visually read, flow-through immunoassay which detects antibodies to Human Immunodeficiency Virus (HIV) Type 1 and Type 2 using a drop of human fingerstick blood. Other sample types which can be tested are venous whole blood, plasma and serum.

- Test yields results in as little as 60 seconds
- Built-in IgG capture procedural control
- Detects IgM and IgG antibodies
- INSTI works with most testing algorithms

Princeton BioMeditech Corporation BioSign Flu A&B
An in vitro rapid qualitative test that detects influenza type A and type B antigens directly from nasal swab, nasopharyngeal swab and nasal aspirate/wash specimens.

- CLIA WAIVED for nasal swab and nasopharyngeal swab specimens
- Rapid diagnosis for early treatment
- Easy-to-read cassette
- Pre-measured extraction reagent capsule for increased accuracy and ease of use
- Detects H1N1 (Swine Flu)

INSTI Hepatits C Antibody Test
The INSTI® HCV Antibody Test is the world’s first one minute test. It is highly accurate, affordable, and easy-to-use. With IgG and IgM procedural control and the ability to test for HCV genotypes 1 – 6, the INSTI HCV Antibody Test is an excellent tool in the fight against Hepatitis C. A hepatitis C blood testing is used to determine if someone has ever been infected with the hepatitis C virus. The INSTI hepatitis C blood tests look for antibodies to the hepatitis C Virus in the blood. They are chemicals that are released into the bloodstream when infected.

- Test yields results in as little as 60 seconds
- Whole Blood/Serum/Plasma
- Sensitivity: 100% - Specificity: 99.7%
- Detects IgM and IgG Antibodies
- INSTI works with most testing algorithms
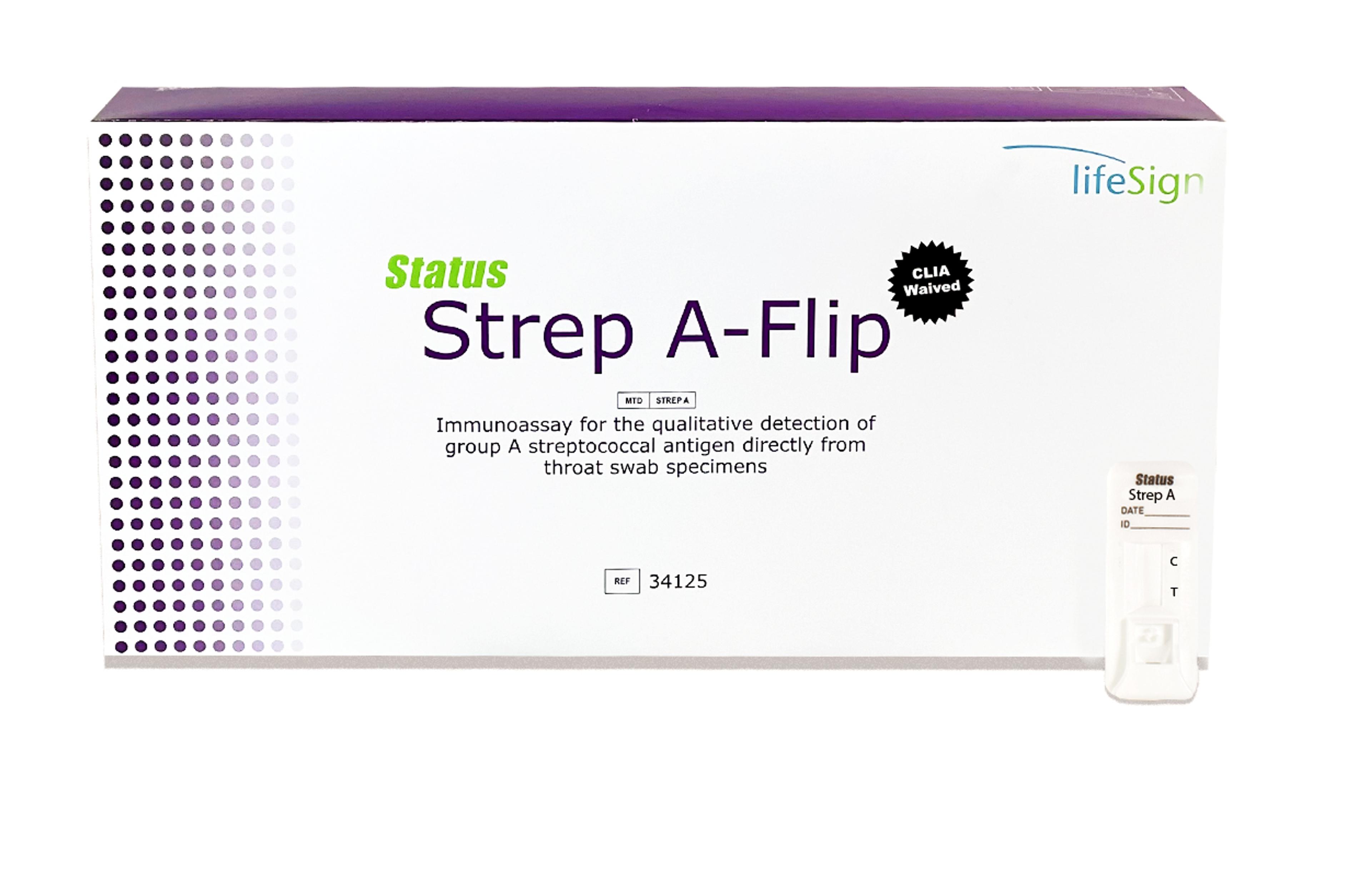
Princeton BioMeditech Corporation Status Strep A Flip
Rapid Detection of Group A Streptococcal Antigen Directly From Throat Swab Specimens

- External positive and negative controls included
- Single use reagent capsules
- Positive results in 5 min
- Sensitivity 96%, specificity 99%
- No counting reagent drops – fewer steps for greater accuracy
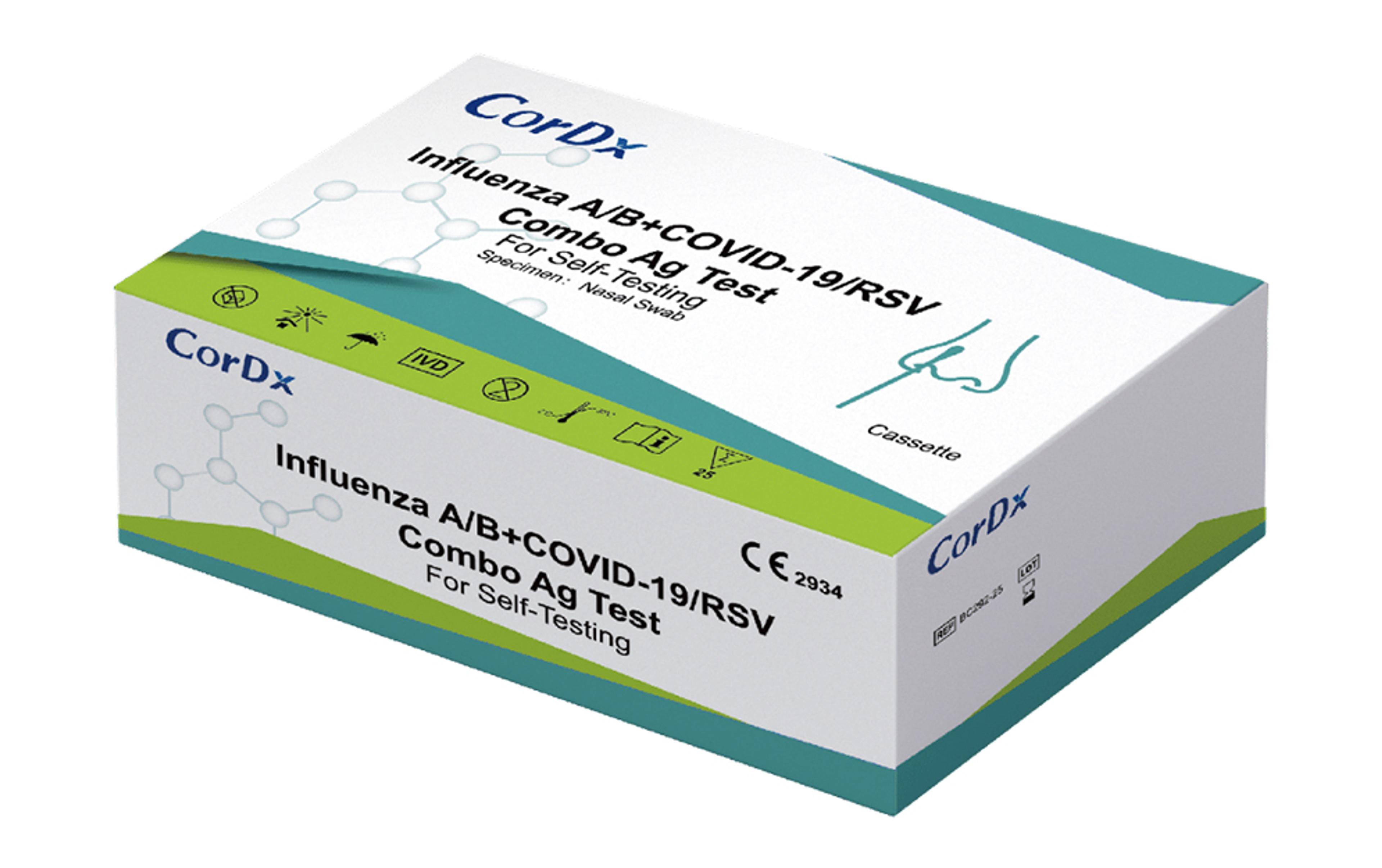
CorDx Influenza A/B + COVID-19/RSV Combo Ag Test
The Influenza A/B+COVID-19/RSV Combo Ag Test (Multi-Panel) is an in vitro immunochromatographic assay for the qualitative and differential detection of nucleocapsid protein antigen from influenza A (including the subtype HIN1), influenza B, RSV and SARS-CoV-2 in nasopharyngeal (NP) fluid specimen.

- Rapid diagnosis of influenza A, influenza B, RSV and SARS-CoV-2 infections
- Multi-Panel display
- For international use
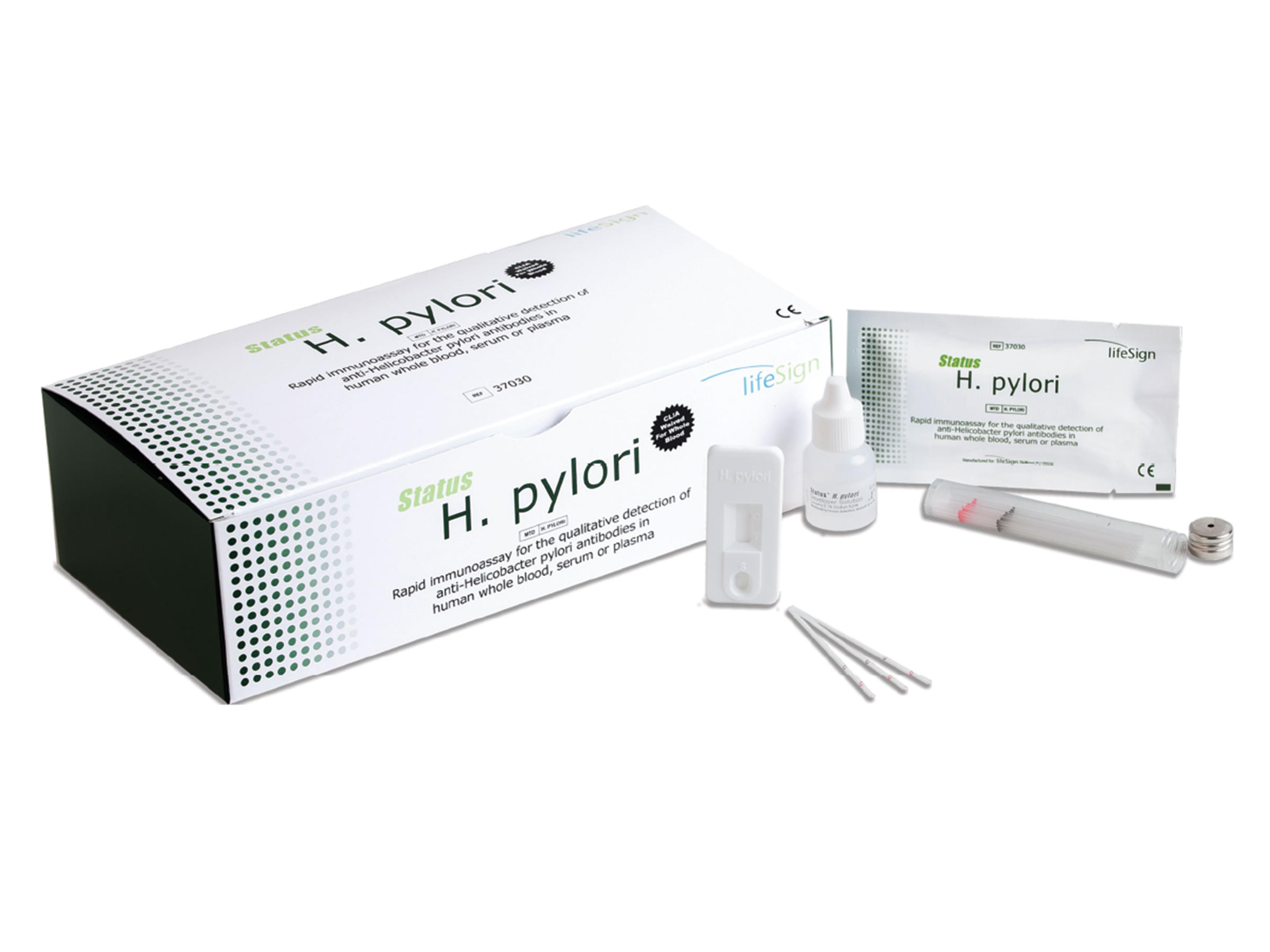
Princeton BioMeditech Corporation BioSign H.pylori IgM/IgG/IgA
For the qualitative detection of anti-Helicobacter pylori antibody in human whole blood, serum, or plasma specimens.

- Requires only 25 μl of whole blood or 10 μl serum/plasma
- Single-reagent procedure
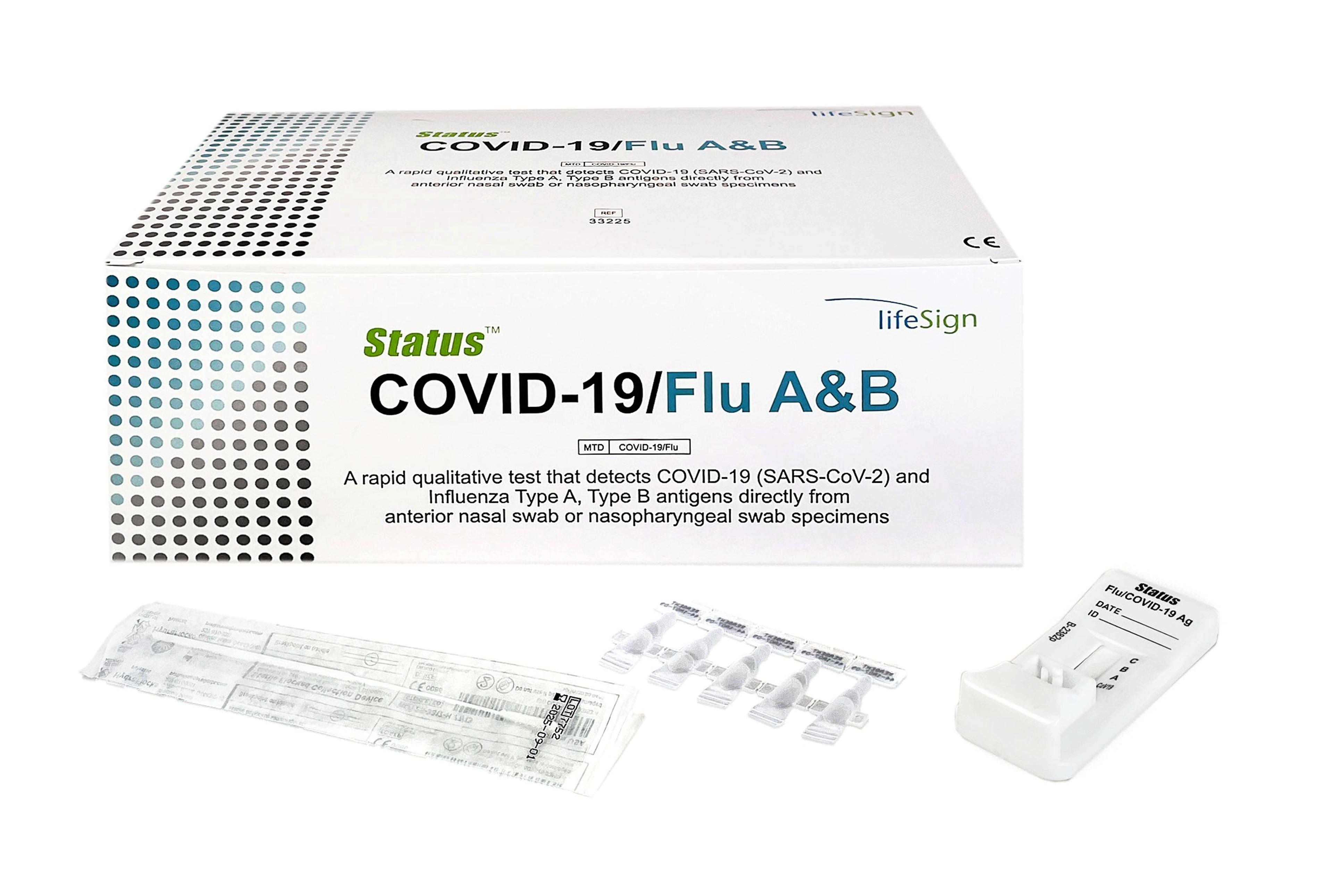
Princeton BioMeditech Corporation Status Covid-19/Flu A&B
A Rapid Immunoassay for the Simultaneous Direct Detection and Differential Diagnosis of SARS-CoV-2, Influenza Type A and Type B Antigen from anterior nasal and nasopharyngeal swab specimens.

- Covid-19: Anterior nasal swab specimen − Sensitivity 93.8 %, Specificity 100%
- Covid-19: Nasopharyngeal − Sensitivity 93.1 %, Specificity 100%
- Flu A: Sensitivity 91.4%, Specificity 95.7%
- Flu B: Sensitivity 87.6%, Specificity 95.9%
- FDA Emergency Use Authorization (EUA)
- Visually read in 15 minutes
- Flocked nasopharyngeal swab for superior specimen collection and patient comfort
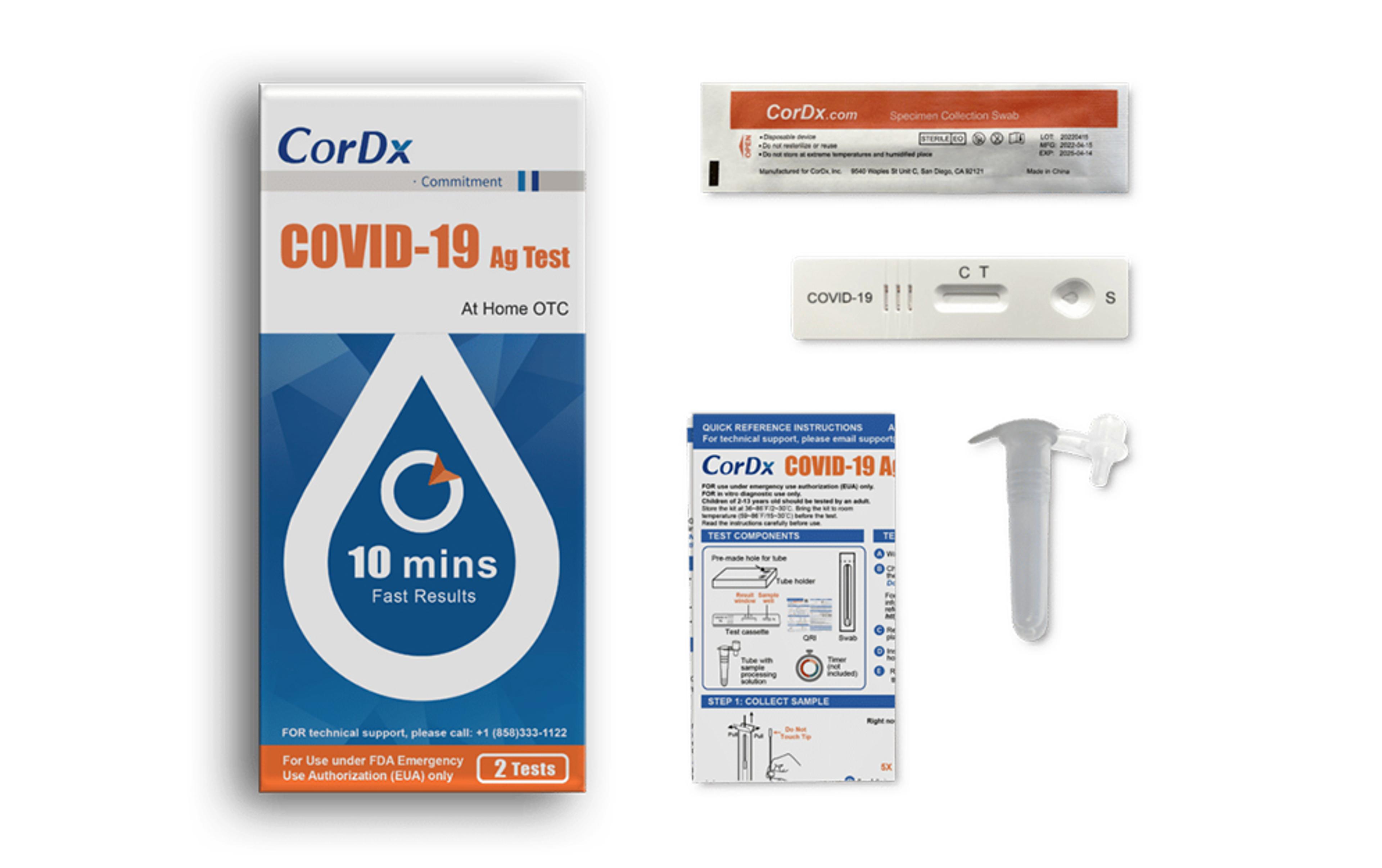
CorDx COVID-19 Ag Test
The CorDx COVID-19 Ag Test is a lateral flow immunoassay device intended for the qualitative detection of nucleocapsid protein antigen from the SARS-CoV-2 virus, delivering results in just 10 minutes.

- Authorized for non-prescription home use with self-collected anterior nasal (nares) swab samples from individuals aged 14 years or older
- Authorized for non-prescription use for adult collected anterior nasal (nares) swab samples from individuals aged two years or older
- This product has not been FDA cleared or approved, but has been authorized by FDA under an EUA
